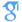X-ray reflectivity measurements are used to determine the configuration of the C2 domain of protein kinase Cα (PKCα-C2) bound to a lipid monolayer of a 7:3 mixture of 1-stearoyl-2-oleoyl-sn-glycero-3-phosphocholine and 1-stearoyl-2-oleoyl-sn-glycero-3-phosphoserine supported on a buffered aqueous solution. The reflectivity is analyzed in terms of the known crystallographic structure of PKCα-C2 and a slab model representation of the lipid layer. The configuration of lipid-bound PKCα-C2 is described by two angles that define its orientation, θ = 35° ± 10° and φ =210° ± 30°, and a penetration depth (=7.5 ± 2 Å) into the lipid layer. In this structure, the β-sheets of PKCα-C2 are nearly perpendicular to the lipid layer and the domain penetrates into the headgroup region of the lipid layer, but not into the tailgroup region. This configuration of PKCα-C2 determined by our x-ray reflectivity is consistent with many previous findings, particularly mutational studies, and also provides what we believe is new molecular insight into the mechanism of PKCα enzyme activation. Our analysis method, which allows us to test all possible protein orientations, shows that our data cannot be explained by a protein that is orientated parallel to the membrane, as suggested by earlier work.
Reference
Chen CH, Málková S, Pingali SV, Long F, Garde S, Cho W and Schlossman ML (). "
Configuration of PKCα-C2 Domain Bound to Mixed SOPC/SOPS Lipid Monolayers
," Biophysical J. , 97(10), 2794-2802
Bibtex
@article{chen2009configuration,
title = {Configuration of PKCα-C2 Domain Bound to Mixed SOPC/SOPS Lipid Monolayers},
author = {Chen, Chiu-Hao and Málková, Šárka and Pingali, Sai Venkatesh and Long, Fei and Garde, Shekhar and Cho, Wonhwa and Schlossman, Mark L},
journal = {Biophysical journal},
volume = {97},
number = {10},
pages = {2794--2802},
year = {2009},
doi = {10.1016/j.bpj.2009.08.037}
}